Strengthening antimicrobial stewardship through a nurse-led program in Mukono District Hospital, Uganda, is a project led by Dr. Stephen Mepham, Dr. Esther Buregyeya, and Dr. Geoffrey Kasirye of Royal Free London NHS, MAKSPH, and THET/CwPAMS.
The focus of the project is promoting antimicrobial stewardship (AMS), as a strategy for containing AMR. Successful AMS implementation is associated with infection control and prevention (IPC) which has been identified as a gap in the hospital, with long periods of stock outs of hand sanitiser. This was in addition to inadequate hand washing facilities and soap, coupled with faulty sinks especially the In-Patient wards (our main focus for the project). Thus, compromising hand hygiene compliance among health workers and patients. The Project embarked on introducing local production of Alcohol Based Hand Rub (ABHR)/Sanitizer at Mukono Hospital, build capacity of health workers and promote sustainability.
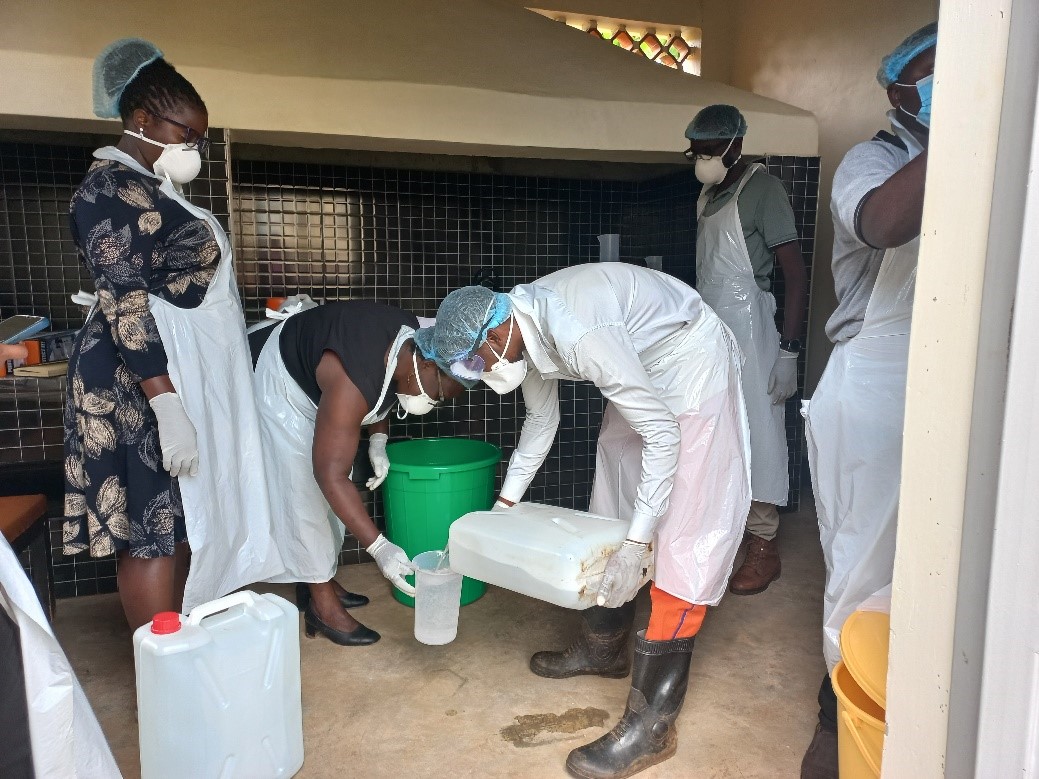
During the World AMR awareness week, 24th November 2023, the MakSPH project team introduced production of ABHR to the general hospital through a CME. Health workers were trained on the role of hand hygiene, controlling health acquired infections and containment of AMR, followed by hands-on training to the AMS committee into the production ABHR.
The training was facilitated by Mr. Fred Twinomugisha, an expert in Infection Prevention and Control (IPC) from Makerere University School of Public Health, this training marked the commencement of the production of the ABHR at the facility. The training was attended by seven health workers from various departments that included pharmacy, maternity ward, laboratory, general ward, and health education. The event aimed to enhance their capacity in the local production of ABHR to improve hand hygiene in the hospital and reduce the hospital associated infections (HAIs).

ABHR production procedure
Every stage of the production process is critical to create an effective and safe hand sanitizer. This process involves the selection of the key ingredients, determining the right concentration, and calculating the precise volumes. These ingredients include absolute alcohol (94%-96%), hydrogen peroxide (3% or 6%), glycerol (98% or 100%) and distilled water.
Production process
During the workshop, Fred Twinomugisha emphasized the significance of striking the right balance in terms of concentration, volumes of various ingredients and a thorough mixing process. This ensures that the chemical reactions yield products that not only meet standards but exceed them guaranteeing the quality of the hand sanitizer.

Safety during the production process
Given the high concentration of the reagents involved in the production process, health workers were informed of the need to protect themselves from harm. Personal Protective Equipment (PPEs), were availed to all, including aprons, eye protection, face masks, hair protection, and gloves. This ensured protection against potential spillages or corrosion from the reagents.
Labelling for the Hand Sanitizer.
The labelling process for the final product was very detailed, including crucial information such as the date of manufacture, expiry, time of manufacture, batch number, and manufacturer details at the facility.

Packaging
The project provided hand held personal 60ml bottles and 1 litre bottles for stationed and at clinic table areas. The stationed bottles were wall mounted to ensure continuity and sustain hand hygiene behaviour. Also to avoid misplaced and lost bottles since they are availed to the patients to increase accessibility.

This workshop not only shed light on the complexities of hand sanitizer production but also highlighted the commitment of Mukono General Hospital administration to continuous improvement and innovation. Within MakSPH, as we continue to prioritize hand hygiene, understanding the intricacies of the products we rely on becomes an empowering and necessary step.
“Dear Makerere university School of public health team, I would like to convey our appreciation to you for spearheading the above training (ABHR production) which was conducted in Mukono General Hospital. We benefitted from the training and hope to put to use the attained skills and apparatus as donated to good use. On a special note, I appreciate Dr. Esther and your team for the coordination. As a hospital we look forward to doing more business with MakSPH, thank you,” appreciation statement from the Medicines and Therapeutic Committee (MTC) at Mukono General Hospital
Armed with newfound knowledge, the health workers pledged to ensure the ongoing production of hand sanitizers and promote their daily use as a steadfast measure to safeguard their health and that of the patients.
This training stands as a testament to the dedication of Mukono General Hospital and its collaborative efforts to fortify hand hygiene practices within the hospital.
We hope that the ABHR will help improve hand hygiene compliance in the health facility.

The available evidence shared in the just concluded National AMR Conference (22-24th Nov, 2023) shows that health facilities are breeding grounds for multidrug drug resistant bacterial strains, which are highly transmissible, highly virulent and with pandemic potential. There is high level of resistance to the 3rd, 4th and 5th generation cephalosporins as well as the reserve drugs like imipenem class. This is because of inappropriate use of antibiotics. In addition to prescribing appropriately, infection prevention and control measures-like hand hygiene and proper waste management are key to breaking the cycle of transmission of these super bags.
Let us encourage health workers to practice proper hand hygiene-observing the FIVE MOMENTS as prescribed by WHO.
Complied by Emmanuel Balinda, Irene Wobusobozi, Esther Buregyeya and Fred Twinomugisha.

